Background: Acute myeloid leukemia (AML) is an aggressive malignancy associated with poor long-term outcomes. This malignancy arises in the context of an immunosuppressive milieu, which fosters immune escape and tumor growth. Myeloid-derived suppressor cells (MDSCs) represent a heterogeneous group of immature myeloid cells with immunosuppressive activity, the most potent of which are the monocytic MDSCs (mMDSCs). The impact of mMDSCs on AML relapse and overall survival has yet to be fully characterized. Therefore, we sought to address this unanswered question through a retrospective analysis of a cohort of AML patients (pts) at Roswell Park Comprehensive Cancer Center.
Methods: Medical records were retrospectively reviewed under an IRB approved protocol to identify pts aged ≥ 18 years old with newly diagnosed AML treated with standard cytarabine and anthracycline based chemotherapy from 2004-2017 at Roswell Park Comprehensive Cancer Center. Demographics, disease-specific variables, baseline clinical characteristics, and treatment response were analyzed using descriptive statistics. Detailed analysis of previously collected clinical multiparameter flow cytometric data was performed utilizing WinList software to identify mMDSCs at serial clinical time points (diagnosis, after induction chemotherapy, and relapse). A mononuclear gate was created utilizing CD45 vs. SSC (blasts excluded), followed by FSC vs. SSC to eliminate dead cells and aggregates. mMDSCs were defined as the subset of marrow cells co-expressing CD14+ and HLA-DR dim and was reported as the percentage of total monocytes in the marrow aspirate sample. mMDSC populations were dichotomized into “Low” and “High” groups based on the median value of the entire cohort. Overall survival (OS) and relapse-free survival (RFS) were estimated utilizing Kaplan-Meier (KM) analysis.
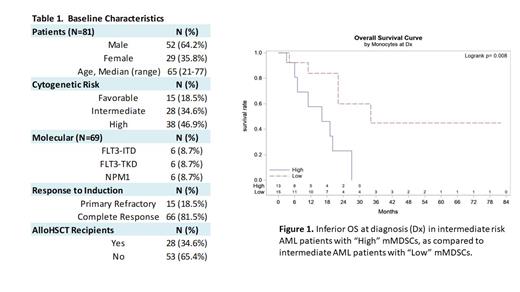
Results: 81 pts with newly diagnosed AML who received induction with a cytarabine and anthracycline based regimen were identified. Median age was 55 years (range 20 - 85), with 52 men (64.2%) (Table 1). Cytogenetics per ELN 2022 were high risk in 38 pts (46.9%), intermediate risk in 28 pts (34.6%), and favorable risk in 15 pts (18.5%). 6/69 (8.7%) pts had FLT3-ITD mutations. Following induction therapy, 15 pts (18.5%) had primary refractory disease with 66 (81.5%) achieving complete remission (CR). 28 pts (34.6%) underwent allo HSCT. Pts with intermediate risk disease and “High” mMDSCs at time of diagnosis and post induction chemotherapy had inferior OS compared to those with “Low” mMDSCs (16 vs. 34 months, P=0.008; 16 vs. 27 months, P=0.03) (Figure 1). There were no significant differences in RFS in intermediate or poor risk pts, and no difference in OS in poor risk pts when comparing “Low” vs. “High” mMDSCs at any time point. Mean duration of follow up was 23.7 months (range 1 - 148 months). Analysis of favorable risk cohort is ongoing.
Conclusions: This retrospective analysis suggests that high numbers of marrow mMDSCs are associated with poor overall survival in pts with intermediate risk AML receiving intensive induction therapy. Of note, other risk factors for poor risk disease such as complete molecular profiling were not available. However, our findings support a potential role of mMDSCs in promoting disease recurrence. Additional studies to further quantify and delineate the biological role of mMDSCs in a larger pt cohort are needed to corroborate these findings and determine the potential role of these immune cells in therapy resistance in AML.
Disclosures
Przespolewski:Jazz Pharmaceuticals: Research Funding. Wang:Sumitomo: Consultancy; Sellas: Consultancy; Rigel: Consultancy; PharmaEssentia: Consultancy; NuProbe: Consultancy; Pfizer: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Kura Oncology: Consultancy; Kite: Consultancy; Johnson and Johnson: Consultancy; Jazz: Consultancy; Janssen: Consultancy; Glaxo Smith Kline: Consultancy; Gilead: Consultancy; Daiichi Sankyo: Consultancy; CTI Biopharma: Consultancy; Bristol Myers Squibb: Consultancy; Astellas: Consultancy, Speakers Bureau; Abbvie: Consultancy; Amgen: Consultancy.